
CAC2 Childhood Cancer Community News Digest (April 14-20)
Assorted News from the Last Week: CAC2 Individual Member Robert Dilley blogging about why sustained research funding is essential to progress. AACR issues calls to

Assorted News from the Last Week: CAC2 Individual Member Robert Dilley blogging about why sustained research funding is essential to progress. AACR issues calls to

In CAC2’s April webinar, “The Role of AI from Diagnosis to Treatment for Childhood Cancer,” our speakers explored how Artificial Intelligence is being used in

Assorted News from the Last Week: A pan-Canadian team has developed a new way to quickly find personalized treatments for young cancer patients, by growing

Assorted News from the Last Week: A man who battled childhood cancer has received the first known transplant of sperm-producing stem cells, in a study
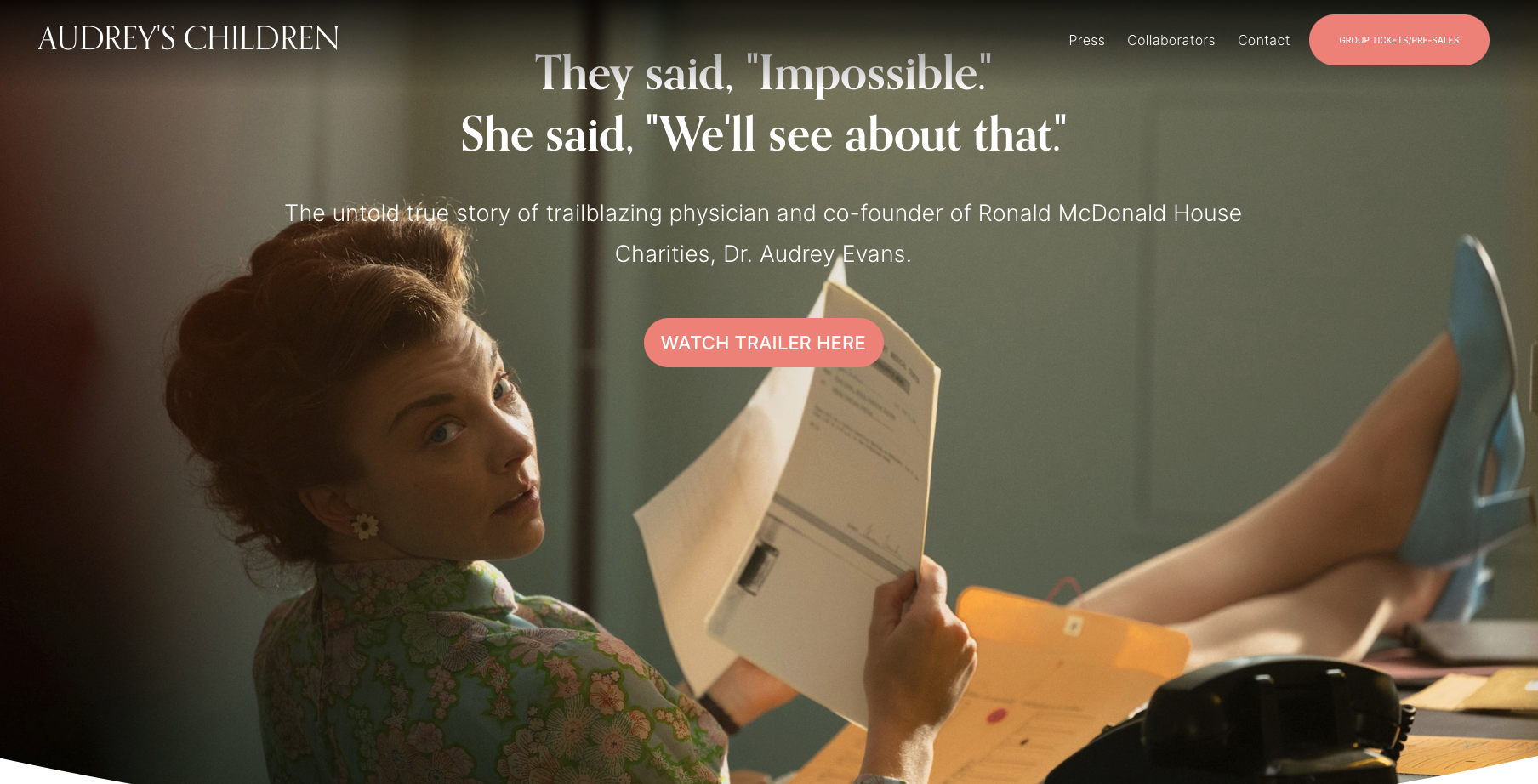
By CAC2 Individual Member Stan Robbins Let’s continue to raise awareness about childhood cancer! AUDREY’S CHILDREN is the extraordinary true story of Dr. Audrey Evans, a pioneering pediatric oncologist

Assorted News from the Last Week: Thanks to all for a fantastic Action Day on February 28. 348 advocates from 40 states participated and shared

The Alliance for Childhood Cancer sends its thanks for a fantastic Action Day on February 28. 348 advocates from 40 states participated and shared their

CAC2 Member Arms Wide Open Childhood Cancer Foundation and Supporting Member The Brain Bodega are launching a pilot program that offers personalized academic support for

Assorted News from the Last Week: The Childhood Cancer Data Initiative (CCDI) is piloting data federation with Kids First Data Resource Center, the Pediatric Cancer

Assorted News from the Last Week: Leo Messi, Alejandra & Richard Gere, Princess Dina supporting OncoThon for childhood cancer research joined the best and the