Supporting Fact Library Graphics
Diagnosis
Note: This section contains facts for four groups: Children (ages 0-14), Children and Adolescents (ages 0-19), Adolescents (ages 15-19), Adolescents and Young Adults (Ages 15-39)
- Childhood and Adolescent cancer (ages 0-19) is not one disease - there are more than 12 major types of pediatric cancers and over 100 subtypes.1 Centre international de recherche sur le cancer, Organisation mondiale de la santé, eds. Paediatric Tumours. 5th ed. International agency for research on cancer; 2022.
- One in 264 children & adolescents is estimated to be diagnosed with cancer before the age of 20 years.2 Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- The average age at diagnosis is 10 overall (ages 0 to 19), 6 years old for children (aged 0 to 14), and 17 years old for adolescents (aged 15 to 19)3Howlander N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2018. Based Novemb 2020 SEER Data Submiss. Published online April 2021. https://seer.cancer.gov/archive/csr/1975_2018/index.html, while adults’ median age for cancer diagnosis is 66.4 Age and Cancer Risk. Natl Cancer Inst. 2021;SEER 21 2013–2017, all races, both sexes. https://www.cancer.gov/about-cancer/causes-prevention/risk/age
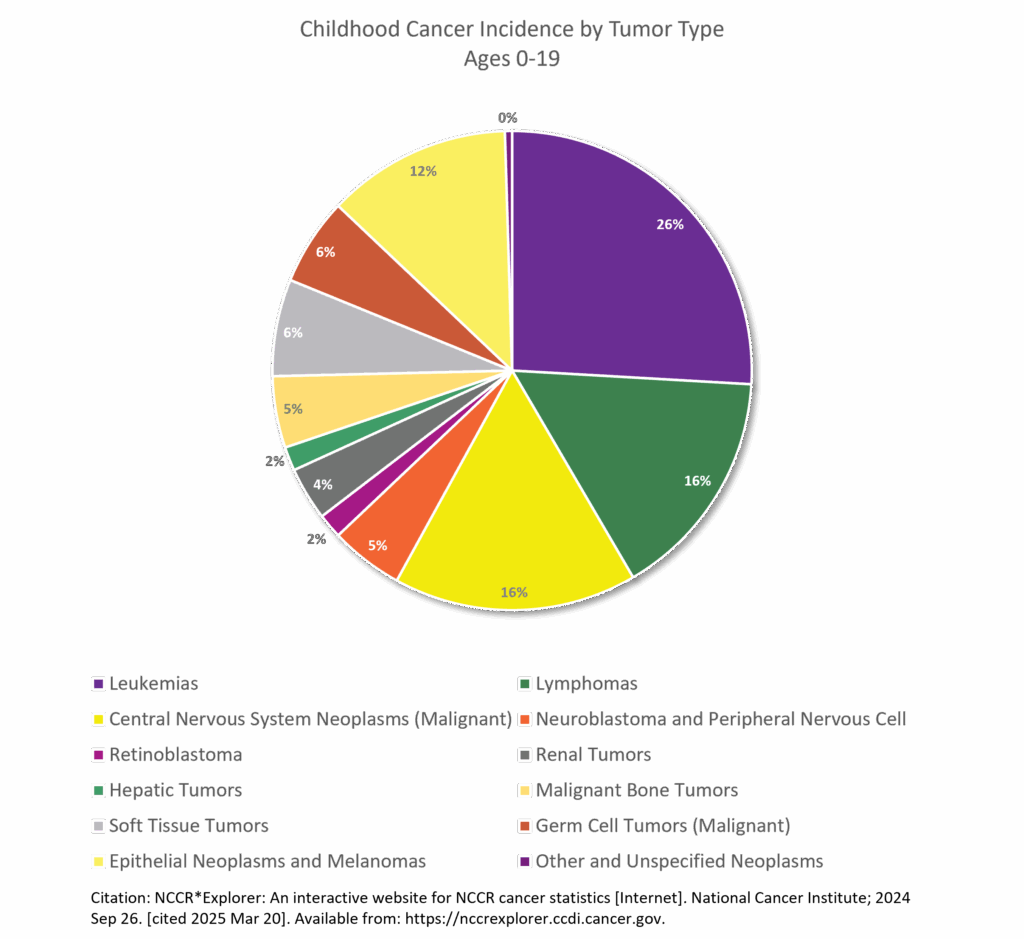
- Leukemia is the most common childhood cancer (ages 0-14), accounting for 28% of cases, followed closely by central nervous system tumors (27%), one-third of which are benign or borderline malignant. In adolescents (ages 15-19), the most common cancer is central nervous system tumors (22%), more than one-half of which are benign or borderline malignant, followed by lymphoma (19%) and leukemia (13%).5 Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- After increasing since at least 1975, the overall invasive cancer incidence rate in children (ages 0-14), declined slightly from 2015 through 2021 by 0.8% per year driven by a recent rapid decline in malignant brain tumors (from 37.3 per million in 2017 to 31.9 per million in 2021) and stabilized rates of lymphoid leukemia. In contrast, overall incidence continued a slow increase in adolescents (by 0.7% per year) because of climbing rates for both lymphoid leukemia and non-Hodgkin lymphoma. Malignant brain tumors decreased rapidly, consistent with the pattern in children, but represent only 9% of all malignancies versus 20% in children.6Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- In 2025, an estimated 9550 children (aged birth to 14 years) and 5140 adolescents (aged 15–19 years) will be diagnosed with cancer.7Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- Approximately 5.7% of newly diagnosed brain tumors, including adults, occur under age 20.8Ostrom QT, Price M, Neff C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022;24(Supplement_5):v1-v95. doi:10.1093/neuonc/noac202
- Childhood brain and other nervous system cancers are most frequently diagnosed among ages 5–9.9Cancer Stat Facts: Childhood Brain and Other Nervous System Cancer (Ages 0–19). SEER 12. Published online 2024. https://seer.cancer.gov/statfacts/html/childbrain.html
- Children with Down syndrome are 10 to 20 times more likely to develop leukemia than children without Down syndrome.10Cancer in Children and Adolescents. Natl Cancer Inst. Published online September 27, 2023. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet
First time Cancer Diagnosis for Adolescents and Young Adults (AYA’s) Ages 15 to 39
- In 2023, it is estimated that there will be 85,980 new cases of cancer among AYAs in the United States. 11 Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15–39). Natl Cancer Inst. Published online 2022. https://seer.cancer.gov/statfacts/html/aya.html
- Overall cancer incidence rates for AYAs increased an average of 0.9% per year between 2014 and 2018. The overall cancer incidence rate was 77.9 cases per 100,000 persons. 12 Cronin KA, Scott S, Firth AU, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer. 2022;128(24):4251-4284. doi:10.1002/cncr.34479
- The most common cancer among AYAs was female breast cancer, which was highest among Black AYAs. 13 Cronin KA, Scott S, Firth AU, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer. 2022;128(24):4251-4284. doi:10.1002/cncr.34479
Survival
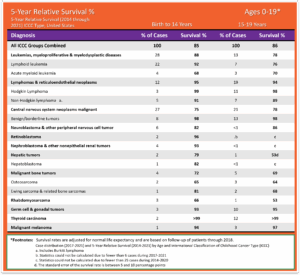
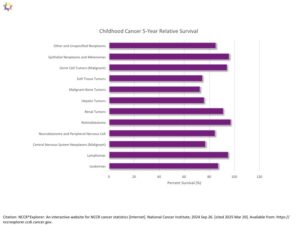
- Cancer survival rates vary not only depending on the type of cancer, but also on individual factors attributable to each child.14 Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for Children and Adolescents With Cancer: Challenges for the Twenty-First Century. J Clin Oncol. 2010;28(15):2625-2634. doi:10.1200/JCO.2009.27.0421 Five-year survival rates can range from almost 0% for cancers such as DIPG (2.2%15Hoffman LM, Veldhuijzen Van Zanten SEM, Colditz N, et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol. 2018;36(19):1963-1972. doi:10.1200/JCO.2017.75.9308), a type of brain cancer, to over 90% for the most common type of childhood cancer known as Acute Lymphoma Leukemia (ALL).16Horton T, Steuber C. Risk group stratification and prognosis for acute lymphoblastic leukemia in children and adolescents. UpToDate. Published online December 29, 2018. https://www.cancer.org/cancer/types/leukemia-in-children/detection-diagnosis-staging/survival-rates.html
- The average 5-year survival rate for childhood cancer (Ages 0-19) as a whole is 86%.17Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- The most common severe or life-threatening chronic health problems related to childhood cancer or its treatment are endocrine disorders such as hypothyroidism or growth hormone deficiency (44%), subsequent neoplasms such as breast cancer or thyroid cancer (7%), and cardiovascular disease such as cardiomyopathy or congestive heart failure, coronary artery disease, and cerebrovascular disease (5.3%).18Bhatia S, Tonorezos ES, Landier W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA. 2023;330(12):1175. doi:10.1001/jama.2023.16875
- Individuals at highest risk for developing treatment-related health problems include patients with brain cancer treated with cranial irradiation (approximately 70% develop severe or life-threatening health problems) and allogeneic hematopoietic stem cell transplant recipients (approximately 60% develop severe or life-threatening health problems).19Bhatia S, Tonorezos ES, Landier W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA. 2023;330(12):1175. doi:10.1001/jama.2023.16875
- Individuals at the lowest risk for developing treatment-related health problems include those who survived solid tumors (such as Wilms tumor) treated with surgical resection alone or with minimal chemotherapy, for whom the prevalence of subsequent health problems is similar to people who did not have cancer during childhood or adolescence.20Bhatia S, Tonorezos ES, Landier W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA. 2023;330(12):1175. doi:10.1001/jama.2023.16875
- Diffuse Intrinsic Pontine Glioma (DIPG) represents approximately 80% of the malignant brainstem tumors occurring in children.21Pai Panandiker AS, Wong JK, Nedelka MA, Wu S, Gajjar A, Broniscer A. Effect of time from diagnosis to start of radiotherapy on children with diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2014;61(7):1180-1183. doi:10.1002/pbc.24971 In the United States, about 300 children are diagnosed with DIPG each year. DIPG primarily affects children between the ages of 5 and 10 years but can occur in younger children and teens. DIPG is rare in adults. 22 Cancer Stat Facts: Childhood Brain and Other Nervous System Cancer (Ages 0–19). SEER 12. Published online 2024. https://seer.cancer.gov/statfacts/html/childbrain.html
- Despite numerous clinical trials, outcomes of children with DIPG continues to remain dismal, with a median survival of only 11 months, while only 10% of DIPG patients have a ≥ 2-year overall survival (OS) rate.23Hoffman LM, Veldhuijzen Van Zanten SEM, Colditz N, et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report From the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol. 2018;36(19):1963-1972. doi:10.1200/JCO.2017.75.9308
- As of January 1, 2020 (the most recent date for which data exist), nearly 496,000 survivors of childhood and adolescent cancer diagnosed at ages 0 to 19 were estimated to be alive in the US. The number of survivors will continue to increase, given the incidence of cancer in children and adolescents has been rising slightly in recent decades and survival rates overall are improving.24Childhood Cancer Survivor Study: An Overview. Natl Cancer Inst. Published online September 7, 2023. https://www.cancer.gov/types/childhood-cancers/ccss
- There are more than 2.1 million cancer survivors diagnosed in the AYA period (ages 15-39) who are living in the United States; most are more than 10 years from diagnosis.25Page LL, Devasia TP, Mariotto A, Gallicchio L, Mollica MA, Tonorezos E. Prevalence of cancer survivors diagnosed during adolescence and young adulthood in the United States. JNCI J Natl Cancer Inst. Published online October 9, 2024:djae250. doi:10.1093/jnci/djae250
Pediatric Cancer 5-Year Relative Survival Percentage, Ages 0 to19 for years 2014 through 2021 for years 2014 through 2021: The table below is a representation of the estimated 5-year survival rates for various types of childhood cancers. It should be noted the survival rates listed below reflect general rates and are in no way a representation of an anticipated actual survival outcome for any individual child. 1 Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
Long Term Health-Effects Associated with Treatments & Survival
- Cancer survival rates vary not only depending on the type of cancer, but also on individual factors attributable to each child. Cancer treatments may harm the body's organs, tissues, or bones and cause health problems later in life. They may include physical, mental, and social problems and second cancers. These health problems are called late effects as a result of surgery, chemotherapy, radiation therapy, and/or stem cell transplant. Late effects in childhood cancer survivors affect the body and mind. Late effects may affect organs, tissues, body function, growth and development. Other late affects are mood, feelings and actions thinking, learning, and memory as well as social and psychological adjustment. Late effects also have a risk of second cancers.26Late Effects of Treatment for Childhood Cancer (PDQ®)–Patient Version. Natl Cancer Inst. Published online February 12, 2025. https://www.cancer.gov/types/childhood-cancers/late-effects-pdq
- The chance of having late effects increases over time. New treatments for childhood cancer have decreased the number of deaths from the primary cancer. Because childhood cancer survivors are living longer, they are having more late effects after cancer treatment. Survivors may not live as long as people who did not have cancer.27Late Effects of Treatment for Childhood Cancer (PDQ®)–Patient Version. Natl Cancer Inst. Published online February 12, 2025. https://www.cancer.gov/types/childhood-cancers/late-effects-pdq
- Overall, children (ages 0-14) and AYA (ages 15-39) cancer survivors were 57% more likely to develop depression, 29% more likely to develop anxiety, and 56% more likely to develop psychotic disorders in the years following treatment compared to their siblings or healthy members of a control group.28Lee ARYB, Low CE, Yau CE, Li J, Ho R, Ho CSH. Lifetime Burden of Psychological Symptoms, Disorders, and Suicide Due to Cancer in Childhood, Adolescent, and Young Adult Years: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023;177(8):790. doi:10.1001/jamapediatrics.2023.2168
- Adult survivors of childhood cancer have a higher risk of developing cognitive impairment later in adulthood.29Phillips NS, Stratton KL, Williams AM, et al. Late-onset Cognitive Impairment and Modifiable Risk Factors in Adult Childhood Cancer Survivors. JAMA Netw Open. 2023;6(5):e2316077. doi:10.1001/jamanetworkopen.2023.16077
- Childhood cancer survivors who received radiation or certain types of chemotherapy have an increased risk of late effects to the heart and blood vessels and related health problems.30Late Effects of Treatment for Childhood Cancer (PDQ®)–Patient Version. Natl Cancer Inst. Published online February 12, 2025. https://www.cancer.gov/types/childhood-cancers/late-effects-pdq
- NCI researchers observed that children who received radiotherapy had an increased risk of developing meningioma, cancer of the membranes that surround the brain and spinal cord meninges.31Withrow DR, Anderson H, Armstrong GT, et al. Pooled Analysis of Meningioma Risk Following Treatment for Childhood Cancer. JAMA Oncol. 2022;8(12):1756. doi:10.1001/jamaoncol.2022.4425
- Long-term survivors of childhood cancer are at substantially elevated risk of treatment-related adverse health effects as they age. For example, one longitudinal study found that 18% of childhood cancer survivors had experienced a major cardiovascular event by the age of 50 years compared with 0.9% of community controls. Thus survivorship care plans are particularly critical for young survivors to help facilitate informed prevention and early detection interventions in addition to surveillance for subsequent cancers.32Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- Children and adolescents (ages 0 to 19) treated more recently, after 1986, may have lower risks of late effects due to modifications in treatment regimens to reduce exposure to radiotherapy and chemotherapy, increased efforts to detect late effects, and improvements in medical care for late effects.33Cancer in Children and Adolescents. Natl Cancer Inst. Published online September 27, 2023. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet
- More than 95% of childhood cancer survivors will have a significant health-related issue by the time they are 45 years of age34Bhatia S, Tonorezos ES, Landier W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA. 2023;330(12):1175. doi:10.1001/jama.2023.16875 ; these health-related issues are late effects of either the cancer or, more commonly, the result of its treatment. 1/3 will suffer severe and chronic side effects; 1/3 will suffer moderate to severe health problems; and 1/3 will suffer slight to moderate side effects35Hudson MM, Ness KK, Gurney JG, et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA. 2013;309(22):2371. doi:10.1001/jama.2013.6296.
- Children and adolescent brain cancer survivors experience significant life-long morbidity from treatment. They have a higher incidence of chronic health conditions including pulmonary fibrosis, cardiac dysfunction, endocrinopathies, neuropathies, and neurocognitive deficits.36Rumberger Rivera L, Springer NL, Bailey K, Patel J, Brett C, Barker E. Opportunities in the translational pipeline for pediatric brain cancer therapies. Pediatr Res. Published online February 1, 2025. doi:10.1038/s41390-025-03847-y.
- Cognitive impairment affects up to one-third of childhood cancer survivors.37Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi:10.3322/caac.21565.
- Long-term survivors of childhood cancer may be at elevated risk for new neurocognitive impairment and decline as they age into adulthood.38Phillips NS, Stratton KL, Williams AM, et al. Late-onset Cognitive Impairment and Modifiable Risk Factors in Adult Childhood Cancer Survivors. JAMA Netw Open. 2023;6(5):e2316077. doi:10.1001/jamanetworkopen.2023.16077.
- A large follow-up study of pediatric cancer survivors found that almost 10% developed a second cancer (most commonly female breast, thyroid, and bone) over the 30-year period after the initial diagnosis.39Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363-385. doi:10.3322/caac.21565.
- Treatment for cancer may cause infertility in childhood cancer survivors. Infertility remains one of the most common and life-altering complications experienced by adults treated for cancer during childhood.40Hudson MM. Reproductive Outcomes for Survivors of Childhood Cancer: Obstet Gynecol. 2010;116(5):1171-1183. doi:10.1097/AOG.0b013e3181f87c4b.
- Having a bone marrow or stem cell transplant usually involves receiving high doses of chemotherapy and sometimes radiation to the whole body before the procedure. In most cases, this permanently stops ovaries from releasing eggs, resulting in lifelong infertility.41How Cancer and Cancer Treatment Can Affect Fertility in Women. Am Cancer Soc Med Editor Content Team. Published online January 17, 2025. https://www.cancer.org/cancer/managing-cancer/side-effects/fertility-and-sexual-side-effects/fertility-and-women-with-cancer/how-cancer-treatments-affect-fertility.html.
- Female childhood cancer survivors who were treated with chemotherapy— even if they did not receive radiation treatments to their chest — are six times more likely than the general population to be diagnosed with breast cancer later in life. For those who did receive chest radiation, that chance increases exponentially and is on par with those who have the BRCA1 or BRCA2 mutations.42Henderson T. Understanding health risks for childhood cancer survivors. Forefr UChicago Med. Published online September 1, 2023. https://www.uchicagomedicine.org/forefront/cancer-articles/understanding-risks-for-childhood-cancer-survivors.
- Childhood cancer survivors are at a 15-fold increased risk of developing Congestive Heart Failure (CHF) and are at a 7-fold higher risk of premature death due to cardiac causes when compared with the general population. There is a strong dose-dependent relation between anthracycline chemotherapy exposure and CHF risk, and the risk is higher among those exposed to chest radiation.43Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123-136. doi:10.1016/S1470-2045(14)70409-7.
- Children who were treated for bone cancer, brain tumors, and Hodgkin lymphoma, or who received radiation to their chest, abdomen, or pelvis, have the highest risk of serious late effects from their cancer treatment, including second cancers, joint replacement, hearing loss, and congestive heart failure.44Cancer in Children and Adolescents. Natl Cancer Inst. Published online September 27, 2023. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet.
- Compared with the general population, survivors of childhood and adolescent cancers have an increased risk of 6 major psychiatric disorders, including: Autism spectrum disorder (hazard ratio [HR], 10.42), ADHD (HR, 6.59), PTSD (HR, 6.10), OCD (HR, 3.37), Major depressive disorder (HR, 1.88), Bipolar disorder (HR, 2.93).45Hsu TW, Liang CS, Tsai SJ, et al. Risk of Major Psychiatric Disorders Among Children and Adolescents Surviving Malignancies: A Nationwide Longitudinal Study. J Clin Oncol. 2023;41(11):2054-2066. doi:10.1200/JCO.22.01189.
- Life expectancy for five-year childhood cancer survivors has steadily increased. Life expectancy for those treated in the 70’s is only 48.5 years and survivors treated in the 80’s have a life expectancy of 53.7 years, while those treated in the 90’s rose to 57.1 years.46Bhatia S, Tonorezos ES, Landier W. Clinical Care for People Who Survive Childhood Cancer: A Review. JAMA. 2023;330(12):1175. doi:10.1001/jama.2023.16875. Normal life expectancy for adults is 77.5 years. 47 Arias E, Kochanek K, Xu J, Tejada-Vera B. Provisional Life Expectancy Estimates for 2022. Centers for Disease Control and Prevention; 2023. https://www.cdc.gov/nchs/data/vsrr/vsrr031.pdf
- Nearly a quarter of childhood cancer survivors experience at least one debilitating neuromuscular condition 20 years post diagnosis.48Rodwin RL, Chen Y, Yasui Y, et al. Longitudinal Evaluation of Neuromuscular Dysfunction in Long-term Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2021;30(8):1536-1545. doi:10.1158/1055-9965.EPI-21-0154
- Nearly 30% of childhood cancer survivors developed pre-diabetes compared with less than 20% of patients in a matched control group. Further analysis showed that between the ages of 40 and 49 years, more than 60% of the survivor group had either pre-diabetes or diabetes.49Dixon SB, Wang F, Lu L, et al. Prediabetes and Associated Risk of Cardiovascular Events and Chronic Kidney Disease Among Adult Survivors of Childhood Cancer in the St Jude Lifetime Cohort. J Clin Oncol. 2024;42(9):1031-1043. doi:10.1200/JCO.23.01005
- Overall, survivors treated with abdominopelvic radiotherapy treatment (ART) were three times more likely to develop a subsequent colorectal cancer (CRC) than those who did not receive ART.50Heymer EJ, Jóźwiak K, Kremer LC, et al. Cumulative Absolute Risk of Subsequent Colorectal Cancer After Abdominopelvic Radiotherapy Among Childhood Cancer Survivors: A PanCareSurFup Study. J Clin Oncol. 2024;42(3):336-347. doi:10.1200/JCO.23.00452
- The North American Children's Oncology Group has developed long-term follow-up guidelines(https://www.cmaj.ca/content/cmaj/suppl/2024/03/05/196.9.E282.DC1/231358-res-4-at.pdf ) to monitor adults who had cancer as children.51Shuldiner J, Sutradhar R, Lau C, et al. Longitudinal adherence to surveillance for late effects of cancer treatment: a population-based study of adult survivors of childhood cancer. Can Med Assoc J. 2024;196(9):E282-E294. doi:10.1503/cmaj.231358
- Childhood cancer survivors have more than a two-fold increased risk of melanoma compared with the general population, and those with an invasive melanoma have more than a two-fold risk of death.52Rotz SJ, Stratton K, Leisenring WM, et al. Melanoma Among Adult Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. Published online January 8, 2025:JCO-24-01519. doi:10.1200/JCO-24-01519
- Compared to the general population, survivors of childhood cancer had a greater association with negative employment transition. There was an association between negative employment transition and chronic health conditions, and individuals with more chronic health conditions had worse transitions.53Berdugo J, Chan A. Chronic health conditions and employment transition in childhood-cancer survivors.
- Childhood cancer survivors have a persistent risk of subsequent leukemia beyond 10 years, especially with high epipodophyllotoxin exposure. Survivors show a 9-fold increased risk for late leukemia and a 5.9-fold risk for very late leukemia compared to the general population. Older age at primary diagnosis, cranial or total body irradiation, and hematopoietic cell transplantation increase leukemia risk.54Ghosh T, Hyun G, Dhaduk R, et al. Late subsequent leukemia after childhood cancer: A report from the Childhood Cancer Survivor Study ( CCSS ). Cancer Med. 2024;13(20):e70086. doi:10.1002/cam4.70086
- Childhood cancer survivors experience fear of recurrence even decades later. Overall, 16.6% of the survivors reported a clinically significant fear of cancer recurrence, and an additional 15.7% reported a high fear of cancer recurrence.55Pizzo A, Leisenring WM, Stratton KL, et al. Fear of Cancer Recurrence in Adult Survivors of Childhood Cancer. JAMA Netw Open. 2024;7(10):e2436144. doi:10.1001/jamanetworkopen.2024.36144
Factors Affecting Follow-up Care
Stakeholders GAO interviewed and studies GAO reviewed identified three factors that affect access to follow-up care for childhood cancer survivors—individuals of any age who were diagnosed with cancer from ages 0 through 19. 2Farb J, Kaczmarek S. Survivors of Childhood Cancer: Factors Affecting Access to Follow-up Care. US Government Accountability Office; 2020. https://www.gao.gov/assets/gao-20-636r.pdf These factors are care affordability, survivors’ and health care providers’ knowledge of appropriate care, and proximity to care. Childhood cancer survivors need access to follow-up care over time for serious health effects known as late effects—such as developmental problems, heart conditions, and subsequent cancers—which result from their original cancer and its treatment.
Affordability: Survivors of childhood cancer may have difficulty paying for follow-up care, which can affect their access to this care. For example, one study found that survivors were significantly more likely to have difficulty paying medical bills and delay medical care due to affordability concerns when compared to individuals with no history of cancer.
Knowledge: Survivors’ access to appropriate follow-up care for late effects of childhood cancer can depend on both survivors’ and providers’ knowledge about such care, which can affect access in various ways, according to stakeholders GAO interviewed and studies GAO reviewed:
- Some survivors may have been treated for cancer at an early age and may have limited awareness of the need for follow- up care.
- Some primary or specialty care providers may not be knowledgeable about guidelines for appropriate follow-up care, which can affect whether a survivor receives recommended treatment. Follow-up care may include psychosocial care (e.g., counseling), and palliative care (e.g., pain management).
Proximity: Survivors may have difficulty reaching appropriate care settings. Stakeholders GAO interviewed and studies GAO reviewed noted that childhood cancer survivors may have to travel long distances to receive follow-up care from multidisciplinary outpatient clinics—referred to as childhood cancer survivorship clinics. The lack of proximity may make it particularly difficult for survivors with limited financial resources to adhere to recommended follow-up care.
Alarmingly, few childhood cancer survivors undergo recommended surveillance for late effects. Only about one-third of survivors adhered to monitoring recommendations for each late effect (cardiac, 36.1%; thyroid, 31.9%; breast, 36.4%). 3Milam J, Kim Y, Roth M, Freyer DR. Late effects surveillance adherence among young adult childhood cancer survivors: A population‐based study. Pediatr Blood Cancer. 2024;71(12):e31328. doi:10.1002/pbc.31328
Treatment & Research
- On average, in 2009 pediatric hospitalizations principally for cancer were 8 days longer and cost nearly 5 times as much as hospitalizations for other conditions (12.0 days versus 3.8 days; $40,400 versus $8,100 per stay). Costs per day were about 70 percent higher for pediatric cancer stays ($3,900 versus $2,300 per day).56 Price R, Stranges E, Elixhauser A. Pediatric Cancer Hospitalizations, 2009. Healthcare Cost & Utilization Project; 2012. https://hcup-us.ahrq.gov/reports/statbriefs/sb132.jsp
- In 2009, pediatric stays principally for cancer cost nearly one billion dollars, accounting for over 5 percent of pediatric non-newborn inpatient hospital costs.57 Price R, Stranges E, Elixhauser A. Pediatric Cancer Hospitalizations, 2009. Healthcare Cost & Utilization Project; 2012. https://hcup-us.ahrq.gov/reports/statbriefs/sb132.jsp
- One in four families lose more than 40% of their annual household income as a result of childhood cancer treatment-related work disruption, while one in three families face other work disruptions such as having to quit work or change jobs.58 The Economic Impact of Childhood Cancer. National Children’s Cancer Society; 2018.
- One in five children who receive a new diagnosis of childhood cancer are already living in poverty.59 The Economic Impact of Childhood Cancer. National Children’s Cancer Society; 2018.
- Parents of long-term childhood cancer survivors reported lower household income and higher risk-of-poverty. In a study group of 383 parents of long-term childhood cancer survivors, 30.4% reported lower household income and were at higher risk-of-poverty.60 Mader L, Roser K, Baenziger J, et al. Household income and risk‐of‐poverty of parents of long‐term childhood cancer survivors. Pediatr Blood Cancer. 2017;64(8):e26456. doi:10.1002/pbc.26456
- A 15-year trend for clinical trials on cellular therapy for children and adolescents (ages 0-19) with cancer in the US concluded that 169 (84%) of 202 trials posted 2007-2022 also included adult populations, only 3 trials enrolled children only. There was no industry funding for CNS tumors.61 Cho S, Miller A, Mosha M, McNerney KO, Metts J. Clinical Trials on Cellular Therapy for Children and Adolescents With Cancer: A 15-Year Trend in the United States. Cureus. 2023;15(10):e47885. doi:10.7759/cureus.47885
- More than 90% of children and adolescents who are diagnosed with cancer each year in the United States are cared for at a children’s cancer center that is affiliated with the NCI-supported Children’s Oncology Group (COG). Children’s Oncology Group is the world’s largest organization that performs clinical research to improve the care and treatment of children and adolescents with cancer. Each year, approximately 4,000 children who are diagnosed with cancer enroll in a COG-sponsored clinical trial. COG trials are sometimes open to individuals aged 29 years or even older when the type of cancer being studied is one that occurs in children, adolescents, and young adults.62 Cancer in Children and Adolescents. Natl Cancer Inst. Published online September 27, 2023. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet
Funding
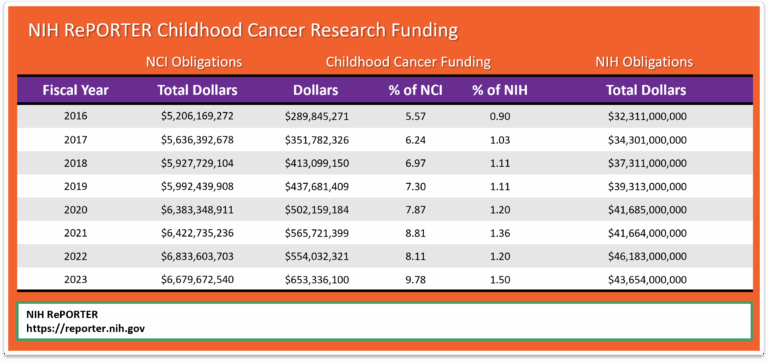
NCI uses the NIH RePORTER, which is a congressionally mandated system all NIH Institutes and Centers (ICs) used to report data by fiscal year (FY). This tool highlights annual support for various Research, Condition, and Disease Categories (RCDC) based on grants, contracts, and other funding mechanisms used across NIH. According to Office of Advocacy Relations, the NIH RePORTER does not account for the totality of NCI’s investment in a given area of research because basic science awards cannot be categorized by individual cancer type. Using Total NCI Obligations, without making allowances for NIH items included in the Pediatric Cancer Amount, would distort the percentage of Total Obligations.
Since we are unable to capture a completely accurate measure of childhood cancer research expenditure as it relates to total research dollars, perhaps a better method to measure progress may be to compare NIH RePORTER pediatric dollars (b) to the Total NIH Dollars (c) for each fiscal year. This method would show changes from one year to the next. Note that the chart below shows growth in pediatric cancer expenditures from 2016 to 2023.
Mortality
- Cancer is the second most common cause of death among children aged 1–14 years (after accidents) and the fourth most common cause of death (after accidents, homicide, and suicide) among adolescents (aged 15–19 years).63Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- On average, about 14% of children die within 5 years of diagnosis, among those children treated in the 70’s and 80’s who survived to five years from diagnosis, 18% of them will die over the next 25 years. In recent decades, cancer treatments have been modified with the goal of reducing life-threatening late effects.64 Armstrong GT, Chen Y, Yasui Y, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374(9):833-842. doi:10.1056/NEJMoa1510795
- 1050 children (aged 0 -14) and 600 adolescents (aged 15-19), are expected to die from cancer in 2025.65Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- It is estimated that in 2023 there were 9,050 deaths due to cancer among AYAs aged 15 to 39.66Cancer Stat Facts: Cancer Among Adolescents and Young Adults (AYAs) (Ages 15–39). Natl Cancer Inst. Published online 2022. https://seer.cancer.gov/statfacts/html/aya.html
- Cancer mortality has declined steadily since 1970, from 6.3 to 1.9 per 100,000 in 2020–2022 in children and from 7.2 to 2.7 in adolescents, for overall reductions of 70% and 63%, respectively.67Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- The 5-year relative survival rate for all cancers combined improved from 58% for diagnoses during the mid-1970s to 85% during 2014 through 2020 in children and from 68% to 87% in adolescents, but it varies substantially by cancer type and age at diagnosis. 68Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin. 2025;75(1):10-45. doi:10.3322/caac.21871
- Between 2015 and 2019, cancer death rates decreased an average of 1.5% per year among children ages 0 to 14, while death rates for AYAs (ages 15 to 39) decreased an average of 0.9% per year.69Cronin KA, Scott S, Firth AU, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer. 2022;128(24):4251-4284. doi:10.1002/cncr.34479
- Brain cancer represents 25% of total childhood cancer deaths while leukemia accounts for 28%.70Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12-49. doi:10.3322/caac.21820
- The median age at death for childhood brain and CNS cancers is age 9 71Cronin KA, Scott S, Firth AU, et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer. 2022;128(24):4251-4284. doi:10.1002/cncr.34479.
- Pediatric brain and other central nervous system tumors are the leading cause of cancer-related fatalities in children and adolescents aged birth to 19 years old. Improvements in mortality in pediatric and adolescent primary central nervous system (CNS) malignancies have lagged behind that of other cancers.72Rumberger Rivera L, Springer NL, Bailey K, Patel J, Brett C, Barker E. Opportunities in the translational pipeline for pediatric brain cancer therapies. Pediatr Res. Published online February 1, 2025. doi:10.1038/s41390-025-03847-y.
- Suicide rates rose for individuals with cancer over the past 20-plus years. Rates of death by suicide per 1000 increased the most among adolescent and young adult men from 4.9 in 2000 to 15.4 in 2021. Deaths by suicide often occurred years after cancer diagnosis — particularly for AYAs (age 15 to 39 years) with thyroid cancer (36.6 per 1,000), testicular cancer (36.3 per 1,000), or melanoma (24.4 per 1,000).73Matsuo K, Duval CJ, Nanton BA, et al. Suicide Deaths Among Adolescent and Young Adult Patients With Cancer. JAMA Netw Open. 2024;7(11):e2442964. doi:10.1001/jamanetworkopen.2024.42964.
- More than half of children with cancer die in the hospital. Researchers from multiple institutions analyzed trends in the place of death for children diagnosed with cancer between 2003 and 2020. About half (52%) died in the hospital, 39.3% died at home, 6.1% died in outpatient medical facilities, 2.2% died in hospice and 0.5% died in nursing facilities.74Jain U, Mathew AT, Jain B, et al. Trends in Location of Death for Individuals With Pediatric Cancer. JAMA Pediatr. 2024;178(11):1221. doi:10.1001/jamapediatrics.2024.3102.
- The most common causes of death in childhood cancer survivors are: The primary cancer comes back. A second (different) primary cancer forms. Heart and lung damage.75Late Effects of Treatment for Childhood Cancer (PDQ®)–Patient Version. Natl Cancer Inst. Published online February 12, 2025. https://www.cancer.gov/types/childhood-cancers/late-effects-pdq.
- Those that survive the five years have an eight times greater mortality rate due to the increased risk of liver and heart disease and increased risk for reoccurrence of the original cancer or of a secondary cancer.76Mertens AC, Liu Q, Neglia JP, et al. Cause-Specific Late Mortality Among 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. JNCI J Natl Cancer Inst. 2008;100(19):1368-1379. doi:10.1093/jnci/djn310.
- There are 69.3 potential life years lost on average when a child dies of cancer49 compared to 12 potential life years lost for adults.77Arias E, Kochanek K, Xu J, Tejada-Vera B. Provisional Life Expectancy Estimates for 2022. Centers for Disease Control and Prevention; 2023. https://www.cdc.gov/nchs/data/vsrr/vsrr031.pdf.
- Survivors of hereditary retinoblastoma, a rare cancer of the eye, have a high risk of developing subsequent cancers, particularly sarcomas of the soft tissue and bone.78Schonfeld SJ, Kleinerman RA, Abramson DH, Seddon JM, Tucker MA, Morton LM. Long-term risk of subsequent cancer incidence among hereditary and nonhereditary retinoblastoma survivors. Br J Cancer. 2021;124(7):1312-1319. doi:10.1038/s41416-020-01248-y.
- Despite improvements in 5-year survival, long-term survivors of childhood cancer are at four times the risk of death compared with the general, aging population.79Dixon SB, Liu Q, Chow EJ, et al. Specific causes of excess late mortality and association with modifiable risk factors among survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Lond Engl. 2023;401(10386):1447-1457. doi:10.1016/S0140-6736(22)02471-0.
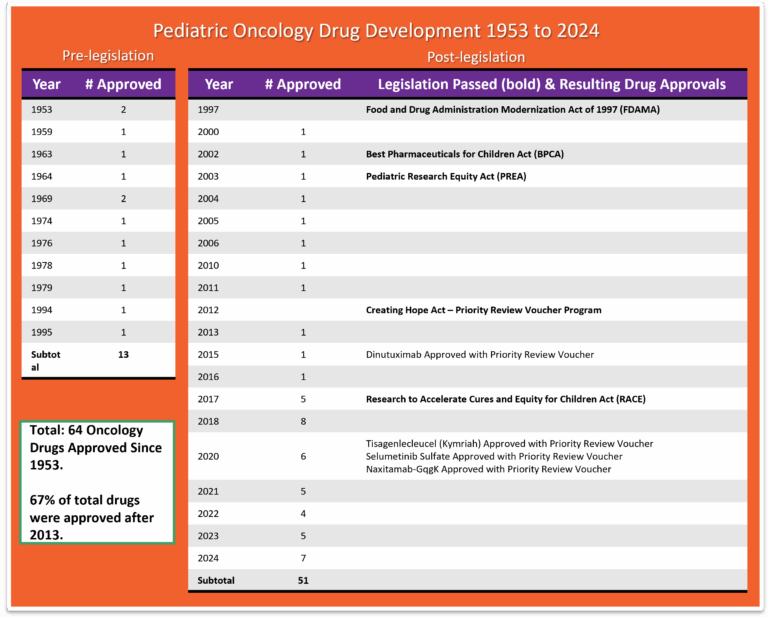
Drug Development
- The FDA awarded Priority Review Vouchers (PRV) for four of the seven drugs originally approved in the first instance for cancer treatment for children. PRV’s are transferable and are desired incentives for developers of drugs for rare pediatric diseases. Holders of a PRV get a faster FDA drug approval process for a future drug of their choice. The vouchers are transferable and may be sold or traded. 80Rare Pediatric Disease Designation and Priority Review Voucher Programs. Office of Orphan Products Development Food and Drug Administration; 2024. https://www.fda.gov/industry/medical-products-rare-diseases-and-conditions/rare-pediatric-disease-designation-and-priority-review-voucher-programs
- The US Congress created the priority review voucher program in 2007 based on a 2006 Health Affairs paper (Ridley et al. 2006). The voucher entitles the bearer to regulatory review in about six months rather than the standard ten months. The Food and Drug Administration (FDA) awards a voucher following approval of a treatment for a neglected disease, rare pediatric disease (Cancer is included in rare pediatric disease) or medical countermeasure. Two drugs receive priority review for each voucher: the drug winning a voucher for a neglected or rare pediatric disease and the drug using a voucher for another indication. The voucher may be sold. For example, a small company might win a voucher for developing a drug for a neglected disease and sell the voucher to a large company for use on a commercial disease. Vouchers can sell for 100’s of millions of dollars.81 Ridley D. Priority Review Vouchers. Accessed March 16, 2025. https://sites.fuqua.duke.edu/priorityreviewvoucher/
- While more than 200 cancer drugs have been developed and approved for adults,82 Benjamin DJ, Xu A, Lythgoe MP, Prasad V. Cancer Drug Approvals That Displaced Existing Standard-of-Care Therapies, 2016-2021. JAMA Netw Open. 2022;5(3):e222265. doi:10.1001/jamanetworkopen.2022.2265 the FDA through December, 2024 has approved a total of 64 drugs for use in the treatment of childhood cancers. 57 of the drugs were originally approved only for adult use, then later for pediatrics also. Today we have seven drugs that were approved in the first instance for use in cancer treatment for children: Teniposide (1992 for ALL) use now discontinued by NCI, clofarabine (2004 for ALL), dinutuximab (2015 for NB), tisagenlecleucel (2017 for ALL), calaspargase pegol-mk (2018 for ALL), selumetinib (2020 for NF1), naxitamab (2020 for NB) and eflornithine (2023 for HRNB). 83 Drugs Approved for Childhood Cancers. Natl Cancer Inst. Published online March 14, 2025. https://www.cancer.gov/about-cancer/treatment/drugs/childhood-cancer-fda-approved-drugs. In addition, the FDA has approved 8 drugs that help to reduce the toxicity associated with certain cancer treatments. 84Biltaji E, Enioutina EY, Yellepeddi V, et al. Supportive care medications coinciding with chemotherapy among children with hematologic malignancy. Leuk Lymphoma. 2020;61(8):1920-1931. doi:10.1080/10428194.2020.1749604
- The median lag time from first-in-human to first-in-child trials of oncology agents that were ultimately approved by FDA was 6.5 years.85 Neel DV, Shulman DS, DuBois SG. Timing of first-in-child trials of FDA-approved oncology drugs. Eur J Cancer Oxf Engl 1990. 2019;112:49-56. doi:10.1016/j.ejca.2019.02.011
- Between the years of 2009 and 2019, nine of the 11 drugs used to treat acute lymphoblastic leukemia — which is the most common childhood cancer — were in and out of shortage.86 Drug Shortages: Root Causes and Potential Solutions. US Food & Drug Administration; 2020. https://www.fda.gov/media/131130/download?attachment
- Researchers found that the probability of FDA drug approval within 10 years was 10.4%, and the probability that development would stall within 10 years was 49.2%.87 Arfè A, Narang C, DuBois SG, Reaman G, Bourgeois FT. Clinical development of new drugs for adults and children with cancer, 2010-2020. JNCI J Natl Cancer Inst. 2023;115(8):917-925. doi:10.1093/jnci/djad082
Global Facts
- Global 5-year net childhood cancer survival is currently estimated at 37.4%.88 Arfè A, Narang C, DuBois SG, Reaman G, Bourgeois FT. Clinical development of new drugs for adults and children with cancer, 2010-2020. JNCI J Natl Cancer Inst. 2023;115(8):917-925. doi:10.1093/jnci/djad082
- In 2017, childhood cancer was the sixth leading cause of total cancer burden globally and the ninth leading cause of childhood disease burden globally. 89 Stiller CA. Global burden of childhood cancer: growing, but controllable. Lancet Oncol. 2019;20(9):1184-1185. doi:10.1016/S1470-2045(19)30424-3
- Globally, in 2017, there were 11.5 million Disability Adjusted Life Years (DALYs) due to childhood cancer, 97.3% were attributable to Years of Life Lost (YLLs). 90 Stiller CA. Global burden of childhood cancer: growing, but controllable. Lancet Oncol. 2019;20(9):1184-1185. doi:10.1016/S1470-2045(19)30424-3
- Approximately 80% of pediatric tumors occur in low- and middle-income countries (LMIC), where diagnostic tools essential for treatment decisions are often unavailable or incomplete. 91 Opoku KB, Santiago T, Kumar P, et al. Transcriptome profiling of pediatric extracranial solid tumors and lymphomas enables rapid low-cost diagnostic classification. Sci Rep. 2024;14(1):19456. doi:10.1038/s41598-024-70541-0
- Approximately 90% of children with cancer live in low-income and middle-income countries (LMICs), where 5-year survival is lower than 20%. 92 Ehrlich BS, McNeil MJ, Pham LTD, et al. Treatment-related mortality in children with cancer in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Oncol. 2023;24(9):967-977. doi:10.1016/S1470-2045(23)00318-2
- Approximately 1 in 15 children receiving cancer treatment in low- and middle-income countries die from treatment-related complications. Although treatment-related mortality has decreased in upper–middle-income countries over time, it remains unchanged in low- and middle-income countries. 93 Ehrlich BS, McNeil MJ, Pham LTD, et al. Treatment-related mortality in children with cancer in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Oncol. 2023;24(9):967-977. doi:10.1016/S1470-2045(23)00318-2
- In 2019, cancer was the fourth leading cause of death and tenth leading cause of Disability-Adjusted Life Years (DALYs) in adolescents and young adults (ages 15 to 39) globally. 94GBD 2019 Adolescent Young Adult Cancer Collaborators. The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022;23(1):27-52. doi:10.1016/S1470-2045(21)00581-7
- When the overall disease burden is studied within the age range encompassing adolescents and young adults (aged 15 years to 39 years), the global burden of cancer contributed more Disability-Adjusted Life Years (DALYs), a combination of Years of Life Lost (YLLs) and Years Lived with Disability (YLDs), to the global disease burden than some high-profile communicable diseases such as HIV/AIDS and sexually transmitted infections.95GBD 2019 Adolescent Young Adult Cancer Collaborators. The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022;23(1):27-52. doi:10.1016/S1470-2045(21)00581-7
- In 2019, 43% (172,000 of 397,000) of childhood cancer cases were undiagnosed globally.96Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20(4):483-493. doi:10.1016/S1470-2045(18)30909-4
- Cancer kills more than 100,000 children (birth to 19) each year, and yet 80% of pediatric cancers are curable with currently available interventions. Notably, the majority of these deaths occur in low‐income and middle-income countries where children have poor access to health services.97Atun R, Bhakta N, Denburg A, et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol. 2020;21(4):e185-e224. doi:10.1016/S1470-2045(20)30022-X
- In Europe, since 1995, a total of 16 drugs have been approved for pediatric cancers. Seven of the 16 have been approved in the first instance specifically for pediatric cancers. Nine of the 16 were first approved for adults, then later for use in pediatric cancer. Eight of the total 16 drugs affected cancers responsible for less than 6% of all European childhood cancer deaths.98Vassal G, de Rojas T, Pearson ADJ. Impact of the EU Paediatric Medicine Regulation on new anti-cancer medicines for the treatment of children and adolescents. Lancet Child Adolesc Health. 2023;7(3):214-222. doi:10.1016/S2352-4642(22)00344-3
- In Europe, cancer is diagnosed in 1 in 350 children before age 15 years and is the leading disease-related cause of death in childhood after infancy.99Søegaard SH, Andersen MM, Rostgaard K, et al. Exclusive Breastfeeding Duration and Risk of Childhood Cancers. JAMA Netw Open. 2024;7(3):e243115. doi:10.1001/jamanetworkopen.2024.3115
- In 2018, The World Health Organization (WHO) launched the Global Initiative for Childhood Cancer with partners to provide leadership and technical assistance to support governments in building and sustaining high-quality childhood cancer programs. The goal is to achieve at least 60% survival rate globally by 2030, for all children with cancer. This represents an approximate doubling of the current cure rate and will save an additional one million lives over the next decade. The objectives are to increase capacity of countries to deliver best practices in childhood cancer care and also to prioritize childhood cancer and increase available funding at the national and global levels.100Childhood Cancer. World Health Organization; 2025. https://www.who.int/news-room/fact-sheets/detail/cancer-in-children
- Some cancers are more prevalent in developing countries. For example, Burkitt’s lymphoma is more common in East and West Africa with over 4,000 cases in East Africa and over 10,000 in West Africa while only around 20 were recorded in the UK in 2015.101Childhood Cancer. World Health Organization; 2025. https://www.who.int/news-room/fact-sheets/detail/cancer-in-children
- Because most of the world's population is NOT covered by cancer surveillance systems or vital registration found in developed countries, and in addition, childhood cancer is rare and often presents with non-specific symptoms that mimic those of more prevalent infectious and nutritional conditions found in many low-income developing countries. Worldwide/UN-regional cancer incidence is therefore estimated using a Baseline Model (BM) method to quantify the cancer burden in children. It is estimated that there will be 13.7 million cases of childhood cancer between 2020-2050. Unless there are major improvements in diagnosis and treatments, of this, 45% will go undiagnosed and 11.1 million will die if no further investments in interventions are made. The vast majority, almost 85%, will be concentrated in developing countries.102Atun R, Bhakta N, Denburg A, et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol. 2020;21(4):e185-e224. doi:10.1016/S1470-2045(20)30022-X
- Current projections show that Africa will account for nearly 50% of the global childhood cancer burden by 2050.103Moeti M. World Cancer Day 2023. February 4, 2023. https://www.afro.who.int/regional-director/speeches-messages/world-cancer-day-2023
Psychosocial Care
Note: Psychosocial care addresses the effects that cancer treatment has on the mental health and emotional wellbeing of patients, their family members, and their professional caregivers. A single profession alone does not provide psychosocial care: Instead, every patient-healthcare provider interaction provides an opportunity to assess the stressors and concerns of children and their family members.
- Childhood cancer threatens every aspect of the family's life and the possibility of a future, which is why optimal cancer treatment must include psychosocial care.104 Adler NE, Page A, Institute of Medicine (U.S.), eds. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. National Academies Press; 2008.
- The provision of psychosocial care has been shown to yield better management of common disease-related symptoms and adverse effects of treatment such as pain and fatigue.105 Jacobsen PB, Holland JC, Steensma DP. Caring for the whole patient: the science of psychosocial care. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(11):1151-1153. doi:10.1200/JCO.2011.41.4078
- Depression and other psychosocial concerns can affect adherence to treatment regimens by impairing cognition, weakening motivation, and decreasing coping abilities.106Świątoniowska-Lonc N, Tański W, Polański J, Jankowska-Polańska B, Mazur G. Psychosocial Determinants of Treatment Adherence in Patients with Type 2 Diabetes - A Review. Diabetes Metab Syndr Obes Targets Ther. 2021;14:2701-2715. doi:10.2147/DMSO.S308322
- For children and families, treating the pain, symptoms, and stress of cancer enhances quality of life and is as important as treating the disease.107National Cancer Policy Forum, Board on Health Care Services, Institute of Medicine, The National Academies of Sciences, Engineering, and Medicine. Comprehensive Cancer Care for Children and Their Families: Summary of a Joint Workshop by the Institute of Medicine and the American Cancer Society. National Academies Press (US); 2015. Accessed March 16, 2025. http://www.ncbi.nlm.nih.gov/books/NBK305219/
- Childhood cancer survivors reported higher rates of pain, fatigue, and sleep difficulties compared with siblings and peers, all of which are associated with poorer quality of life.108Armenian SH, Landier W, Hudson MM, Robison LL, Bhatia S, COG Survivorship and Outcomes Committee. Children’s Oncology Group’s 2013 blueprint for research: survivorship and outcomes. Pediatr Blood Cancer. 2013;60(6):1063-1068. doi:10.1002/pbc.24422
- Changes in routines disrupt day-to-day functioning of siblings.109Alderfer MA, Long KA, Lown EA, et al. Psychosocial adjustment of siblings of children with cancer: a systematic review. Psychooncology. 2010;19(8):789-805. doi:10.1002/pon.1638 76 Siblings of children with cancer are at risk for emotional and behavioral difficulties, such as anxiety, depression, and post traumatic stress disorder. 110Alderfer MA, Labay LE, Kazak AE. Brief report: does posttraumatic stress apply to siblings of childhood cancer survivors? J Pediatr Psychol. 2003;28(4):281-286. doi:10.1093/jpepsy/jsg016
- Symptoms of posttraumatic stress disorder are well documented for parents whose children have completed cancer treatment.111Kazak AE, Alderfer M, Rourke MT, Simms S, Streisand R, Grossman JR. Posttraumatic stress disorder (PTSD) and posttraumatic stress symptoms (PTSS) in families of adolescent childhood cancer survivors. J Pediatr Psychol. 2004;29(3):211-219. doi:10.1093/jpepsy/jsh022
- Chronic grief has been associated with many psychological (e.g., depression and anxiety) and somatic symptoms (e.g., loss of appetite, sleep disturbances, fatigue), including increased mortality risk.112Alam R, Barrera M, D’Agostino N, Nicholas DB, Schneiderman G. Bereavement Experiences of Mothers and Fathers Over Time After the Death of a Child Due to Cancer. Death Stud. 2012;36(1):1-22. doi:10.1080/07481187.2011.553312
- Financial hardship during childhood cancer has been found to affect a significant proportion of the population and to negatively impact family wellbeing.113Bona K, Dussel V, Orellana L, et al. Economic impact of advanced pediatric cancer on families. J Pain Symptom Manage. 2014;47(3):594-603. doi:10.1016/j.jpainsymman.2013.04.003
- Adolescents with cancer experienced significantly more Health Related Hindrance (HRH) of personal goals than healthy peers, and their HRH was significantly associated with poorer health-related quality of life, negative affect, and depressive symptoms.114Schwartz LA, Brumley LD. What a Pain: The Impact of Physical Symptoms and Health Management on Pursuit of Personal Goals Among Adolescents with Cancer. J Adolesc Young Adult Oncol. 2017;6(1):142-149. doi:10.1089/jayao.2016.0031
- Peer relationships of siblings of children with cancer are similar to classmates, though they experience small reductions in activity participation and school performance.115Alderfer MA, Stanley C, Conroy R, et al. The social functioning of siblings of children with cancer: a multi-informant investigation. J Pediatr Psychol. 2015;40(3):309-319. doi:10.1093/jpepsy/jsu079
- Chronic health conditions resulting from childhood cancer therapies contribute to emotional distress in adult survivors.116Vuotto SC, Krull KR, Li C, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123(3):521-528. doi:10.1002/cncr.30348
- Parents have been found to report significant worsening of all their own health behaviors, including poorer diet and nutrition, decreased physical activity, and less time spent engaged in enjoyable activities 6 to 18 months following their child’s diagnosis.117Wiener L, Viola A, Kearney J, et al. Impact of Caregiving for a Child With Cancer on Parental Health Behaviors, Relationship Quality, and Spiritual Faith: Do Lone Parents Fare Worse? J Pediatr Oncol Nurs Off J Assoc Pediatr Oncol Nurses. 2016;33(5):378-386. doi:10.1177/1043454215616610
Prevention
- Any substance that causes cancer is known as a carcinogen. But simply because a substance has been designated as a carcinogen does not mean that the substance will necessarily cause cancer. Many factors influence whether a person exposed to a carcinogen will develop cancer, including the amount and duration of the exposure and the individual’s genetic background. 118 Environmental Carcinogens and Cancer Risk. Natl Cancer Inst. Published online April 6, 2023. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/carcinogens
- Cancers caused by involuntary exposures to environmental carcinogens are most likely to occur in subgroups of the population, such as workers in certain industries who may be exposed to carcinogens on the job. 119 Environmental Carcinogens and Cancer Risk. Natl Cancer Inst. Published online April 6, 2023. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/carcinogens
- Two organizations—the National Toxicology Program (NTP), an interagency program of the U.S. Department of Health and Human Services (HHS), and the International Agency for Research on Cancer (IARC), the cancer agency of the World Health Organization—have developed lists of substances that, based on the available scientific evidence, are known or are reasonably anticipated to be human carcinogens. 120 Environmental Carcinogens and Cancer Risk. Natl Cancer Inst. Published online April 6, 2023. https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/carcinogens
- The National Toxicology Program (NTP) cumulative report now includes 256 listings of substances — chemical, physical, and biological agents; mixtures; and exposure circumstances — that are known or reasonably anticipated to cause cancer in humans. The latest report, the 15th Report on Carcinogens was released on December 21, 2021.121 National Toxicology Program (NTP). 15th RoC Dashboard. Published online November 2021:ROC-15. doi:10.22427/NTP-DATA-ROC-15
- Childhood cancer is fundamentally different to adult cancer in its biology, clinical classification, and treatment. Most childhood cancers are not caused by modifiable risk factors, public health campaigns would not have a large effect on decreasing their incidence.122Valery PC, McBride CA. Sustainable care for indigenous children with cancer. Lancet Oncol. 2020;21(4):489-491. doi:10.1016/S1470-2045(20)30137-6
- Over the past 50 years, the use of artificial chemicals in products has increased exponentially. Most of these chemicals were not tested for safety before widespread use, and the impacts of exposures are just now being realized. Children are especially vulnerable to the health impacts of chemical exposures, and these exposures are now known to be an important component of rising rates of diseases such as asthma, some cancers, and neurodevelopmental disorders in children.123Huffling K, McLaughlin J. Pediatric Chemical Exposure: Opportunities for Prevention. J Pediatr Health Care. 2022;36(1):27-33. doi:10.1016/j.pedhc.2021.09.009
- Children are at an elevated risk for chronic disease because of increased exposure to environmental toxins. The U.S. Environmental Protection Agency (EPA) (2017) identifies children as uniquely vulnerable to environmental risks because of rapidly developing brains, lungs, immune and other bodily systems with less developed natural defenses than adults, including more permeable blood-brain barriers, and metabolic and detoxification pathways that are not yet fully developed.124Huffling K, McLaughlin J. Pediatric Chemical Exposure: Opportunities for Prevention. J Pediatr Health Care. 2022;36(1):27-33. doi:10.1016/j.pedhc.2021.09.009
- Phthalates are a class of chemicals found in a variety of products. They are mixed with polyvinyl chloride and other plastics as a plasticizer that helps to make them soft and flexible. They are also added to cosmetics and other personal care products (often as a fragrance stabilizer), in medical equipment and coatings on medications, food production equipment and packaging, flooring, wall coverings, and other home products.125Huffling K, McLaughlin J. Pediatric Chemical Exposure: Opportunities for Prevention. J Pediatr Health Care. 2022;36(1):27-33. doi:10.1016/j.pedhc.2021.09.009 In a study of 1.3 million children aged under 19 years of age, childhood phthalate exposure was associated with incidence of osteosarcoma and lymphoma. 126Ahern TP, Spector LG, Damkier P, et al. Medication-Associated Phthalate Exposure and Childhood Cancer Incidence. J Natl Cancer Inst. 2022;114(6):885-894. doi:10.1093/jnci/djac045
- Pesticides are a group of chemicals intended to kill unwanted insects, plants, molds, and rodents, making them inherently toxic chemicals. Pesticides are not species-specific in their neurotoxic properties—a wanted effect on the nervous system of an insect can also be an unwanted effect on the nervous system of a child.127Huffling K, McLaughlin J. Pediatric Chemical Exposure: Opportunities for Prevention. J Pediatr Health Care. 2022;36(1):27-33. doi:10.1016/j.pedhc.2021.09.009 Exposures to pesticides, tobacco smoke, solvents, and traffic emissions have consistently demonstrated positive associations with risk of developing childhood leukemia.128Whitehead TP, Metayer C, Wiemels JL, Singer AW, Miller MD. Childhood Leukemia and Primary Prevention. Curr Probl Pediatr Adolesc Health Care. 2016;46(10):317-352. doi:10.1016/j.cppeds.2016.08.004
- Researchers found a higher level of common household pesticides in the urine of children with acute lymphoblastic leukemia. The findings should not be seen as cause-and-effect, but suggests an association between pesticide exposure and development of childhood ALL.129Soldin OP, Nsouli-Maktabi H, Genkinger JM, et al. Pediatric acute lymphoblastic leukemia and exposure to pesticides. Ther Drug Monit. 2009;31(4):495-501. doi:10.1097/FTD.0b013e3181aae982
- The U.S. Environmental Protection Agency (EPA) reports that 75 percent of U.S. households used at least one pesticide product indoors during the past year. The EPA also states, “Exposure to pesticides may result in irritation to eye, nose and throat, damage to central nervous system, kidney and increased risk of cancer.”130Pesticides’ Impact on Indoor Air Quality. United States Environmental Protection Agency; 2024. https://www.epa.gov/indoor-air-quality-iaq/pesticides-impact-indoor-air-quality
- Exposure to toxic substances, such as industrial chemicals and radiation, can increase the risk of leukemia. People may encounter radiation during imaging tests such as MRI scans, X-rays, and CT scans.131Schmidt JA, Hornhardt S, Erdmann F, et al. Risk Factors for Childhood Leukemia: Radiation and Beyond. Front Public Health. 2021;9:805757. doi:10.3389/fpubh.2021.805757
- Since children are more radiosensitive than adults and although CT scans are very useful clinically, potential cancer risks exist from associated ionizing radiation.132Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet Lond Engl. 2012;380(9840):499-505. doi:10.1016/S0140-6736(12)60815-0
- Exposure of parents to ionizing radiation is also a possible concern in terms of the development of cancer in their future offspring. Children whose mothers had x-rays during pregnancy (that is children who were exposed before birth) and children exposed after birth to diagnostic medical radiation from computed tomography (CT) scans have been found to have a slight increase in risk of leukemia and brain tumors and possible other cancers.133Cancer in Children and Adolescents. Natl Cancer Inst. Published online September 27, 2023. https://www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet
- Risk of childhood leukemia was associated with higher crop area near mother’s homes during pregnancy; CNS tumors were associated with higher cattle density.134Patel DM, Gyldenkærne S, Jones RR, et al. Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort. Environ Int. 2020;143:105955. doi:10.1016/j.envint.2020.105955
- Intake of vitamins and folate supplementation during the preconception period or pregnancy has been demonstrated to have a protective effect.135Metayer C, Dahl G, Wiemels J, Miller M. Childhood Leukemia: A Preventable Disease. Pediatrics. 2016;138(Supplement_1):S45-S55. doi:10.1542/peds.2015-4268H
- Researchers used statistical modeling to look at radon levels and cancer in 727 counties around the United States. Even at concentrations below levels where federal officials recommend steps to reduce radon exposure, they saw links between childhood leukemia and radon. The U.S. Environmental Protection Agency says no level of radon is safe and recommends mitigation efforts when concentrations reach 148 Becquerel’s per cubic meter of air.136Bozigar M, Konstantinoudis G, Zilli Vieira CL, et al. Domestic radon exposure and childhood cancer risk by site and sex in 727 counties in the United States, 2001–2018. Sci Total Environ. 2024;954:176288. doi:10.1016/j.scitotenv.2024.176288
Disclaimer
THIS DOCUMENT IS NOT INTENDED TO OFFER SPECIFIC STATISTICS REGARDING AN INDIVIDUAL PATIENT OR THE PATIENT’S SPECIFIC FORM OF CANCER AND IS NOT A SUBSTITUTE FOR INFORMATION THAT MAY BE SOUGHT FROM A PHYSICIAN. IT IS MERELY INTENDED, BASED ON INFORMATION PRESENTLY AVAILABLE TO THE AUTHORS, TO BE A GOOD FAITH GENERAL PRESENTATION OF CHILDHOOD CANCER STATISTICS THAT MAY BE HELPFUL TO OTHERS SEEKING SUCH GENERAL INFORMATION.